Covid vaccines limited to high -risk patients this fall season, says the FDA

NEWYou can now listen to Fox News articles!
The Food and Drug Administration of the United States (FDA) has authorized COVVI-19 COVVI-19 vaccines for the fall-but only for high-risk groups.
The Secretary of Health and Social Services, Robert F. Kennedy, announced on Wednesday the most recent actions of the FDA in an article on X.
“I promised 4 things,” wrote Kennedy. “1. to end the codvised vaccine mandates; 2. to keep the vaccines available to people who wish them, in particular the vulnerable; 3. To demand tests controlled by placebo from companies; 4. To end the emergency.”
The largest epidemic of measles in the United States is officially over, say health officials
“In a series of FDA actions today, we have reached the four objectives.”
In the position, RFK said that the FDA had made a “marketing authorization” for high -risk groups for the following vaccines: modern (6 months and more), Pfizer (5 years and over) and Novavax (12 years and over).
“These vaccines are available for all patients who choose them after consulting their doctors,” RFK wrote.
High -risk groups include people over the age of 65 and those who are more likely to develop a serious covid disease.
The medical group goes against the CDC, recommends photos cobe for young children
On its website, the CDC lists the conditions that can increase the risk of severe covid, in particular asthma, cancer, heart disease, cerebrovascular diseases, diabetes, dementia, mood disorders, obesity, Parkinson’s disease and chronic lung disease, liver or kidneys, among others.
RFK also announced that emergency authorizations for cocovid vaccines had been canceled.
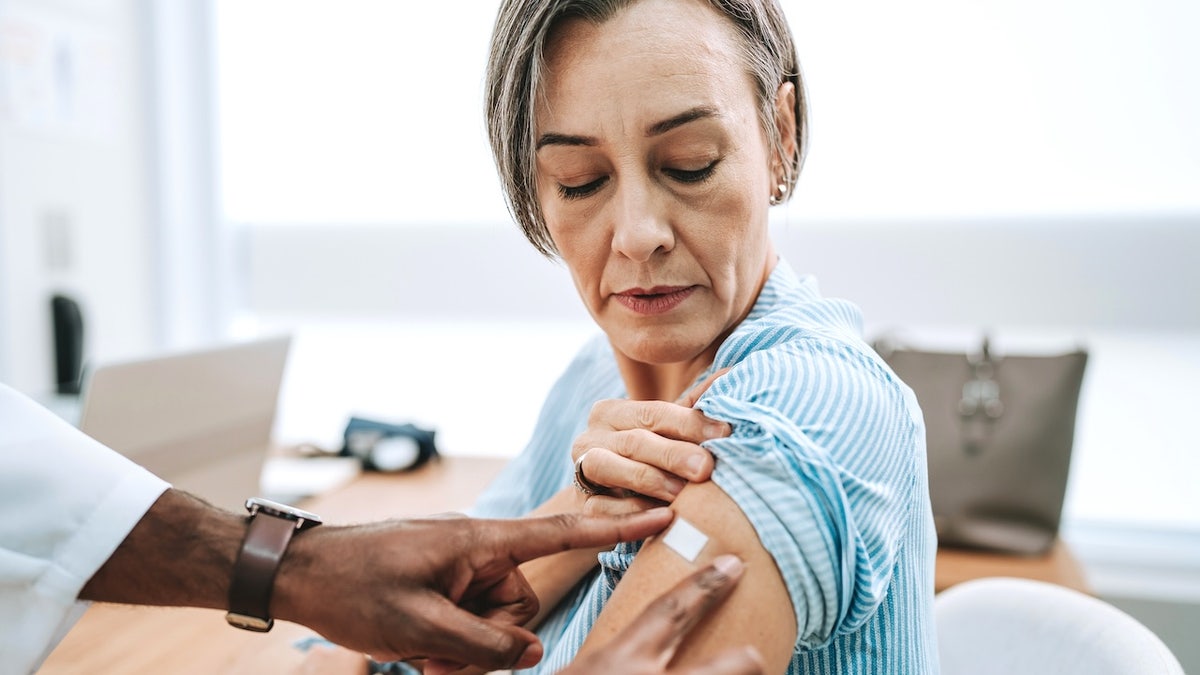
High -risk groups include people over the age of 65 and those who are more likely to develop a serious covid disease. (istock)
“The American people demanded science, security and common sense,” continued RFK. “This frame delivers the three.”
Before this change, the centers for Disease Control and Prevention had recommended the vaccine for all the Americans 6 months and more.
Click here to obtain the Fox News app
In May 2025, Kennedy announced that COVVI-19 vaccines would be withdrawn from the CDC routine immunization calendar for healthy children and pregnant women.
Click here to register for our Health Newsletter
Instead of a universal recommendation, the CDC updated orientations require “shared clinical decision -making”, in which parents and doctors discuss the advantages and risk of vaccination for each individual case.
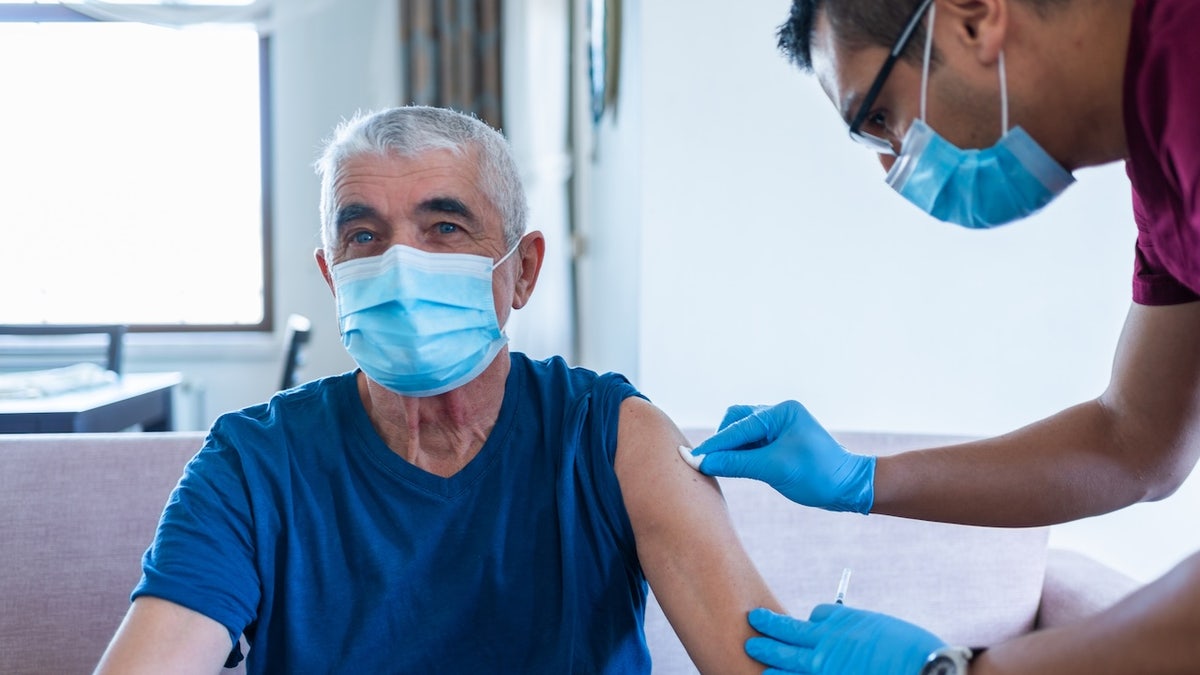
Before this change, the centers for Disease Control and Prevention had recommended the vaccine for all the Americans 6 months and more. (istock)
However, the American Academy of Pediatrics (AAP) still includes it in its annual vaccination calendar, as previously reported by Fox News Digital.
For more health items, visit www.foxnews.com/health
“It should be a conversation between the pediatrician, the patient and the parent, and should depend on the health of the child as well as on the current state of COVID,” said Dr. Marc Siegel, lead medical analyst of Fox News.



